Freyr provides Regulatory Affairs service support to the Innovator medicine companies during IND filing process, starting from pre-IND meetings to IND submission and further to regulatory compliance & maintenance.
Facing issue in account approval? email us at info@ipt.pw
Click to Ckeck Our - FREE SEO TOOLS
Freyr provides Regulatory Affairs services in handling submission of CTA applications/Investigational Medicinal Product Dossier (IMPD) as per EU requirements for different types of medicinal products like New drugs, Recombinant Protein Products, Vaccines, Stem Cell-Based Products etc.
Freyr provides Regulatory Affairs services during planning, preparation and submission of Clinical Trail Application to comply with Health Authority Requirements.
Freyr provides end-to-end Regulatory Affairs services to the Biologics/Biosimilar product companies during BLA submission process, starting from pre-BLA/BPD meetings to further Life Cycle Management (LCM) of the various biological products.
Freyr is a Strategic Regulatory partner providing end-to-end Regulatory solutions and services to Innovator pharma companies during their innovator drug development process.
Freyr provides Swiss Authorized Representative (CH-REP) services to foreign medical device manufacturers for their product registration and market entry in Switzerland and acts as a single point of contact in the Country for liaison with Regulatory Agency.
Freyr provides cosmetic regulatory services in Malaysia that span across cosmetic product registration, classification, Notification, formulation, claims review, CPSR and technical dossier compilation as per NPRA regulation.
Freyr provides food regulatory services in Malaysia that span across food product registration, classification, formulation, ingredient assessment, technical dossier compilation and submission as per NPRA regulations.
Freyr provides medical device regulatory services in Malaysia that span across medical device registration, medical device classification and market entry as per NPRA regulatory requirements.
Freyr provides pharma/Medicinal product regulatory services in Malaysia as per NPRA regulations during Medicinal Product Registration, classification, Market authorization, Dossier Gap analysis and compliant with ACTD.
Freyr provides End-to-End Regulatory Services in Malaysia to the pharmaceuticals, Medical Device, Food Supplements, and Cosmetic Companies to comply with NPRA Regulations.
Freyr iREADY is a technology based Cosmetic Ingredient Database platform and it provides Regulatory compliance support to manufacturers for cosmetics ingredients and management of product formulae in global markets.
Freyr provides End to End Chemical Regulatory Compliance services to health care consumers like product registration, notification, Formulation review and We offer EU REACH and CLP Registration/Dossier Update, Hazard Communication for quick market access.
Freyr Food Regulatory Affairs provides end to end regulatory support for Food/dietary supplements manufacturers in product notification, classification, and registration across the globe.
Freyr provides end to end Cosmetic Regulatory Services like formulation & ingredient review, label review, claims review, safety assessment & toxicology services, dossier compilation and market entry support.
Freyr acts as a Swiss Authorized Representative(CH-REP) on behalf of foreign medical device manufacturers during registration and compliant market entry of their products as per Swissmedic Regulations.
Freyr provides United Kingdom Responsible Person (UKRP) services to foreign medical device manufacturers in product registration and market entry in the UK and acts as a single point of contact in the Country for liaison with Regulatory Agency.
Freyr provides End-to-End Post Brexit regulatory support for Pharma, Medical Devices, Cosmetics and Food Supplements manufacturers who are willing to market their products in United Kingdom (UK) and EU.
Freyr provides end to end regulatory support for medical device & IVD manufacturers in product registration, notification, classification across the globe.
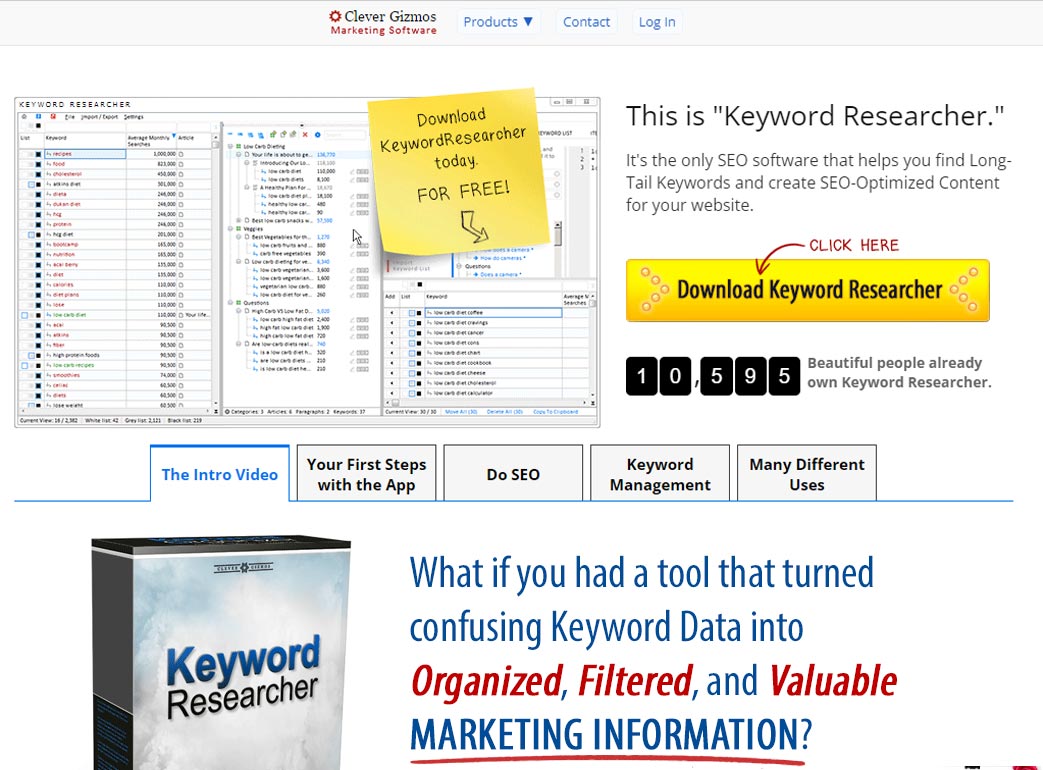
Freyr SUBMIT Pro is one of the best eCTD software tool which helps Life science companies in various regulatory submissions to global health Authorities.